Data & Updates

The use of animal free collagen could significantly lower the development costs of a medical device
The use of animal free recombinant collagen, rather than collagen extracted from animals, could significantly lower the development costs of a medical device by not contributing to the device regulatory classification so that the device is deemed Class II rather than an automatic Class III. Read the news release here: Mobile users click the link […]
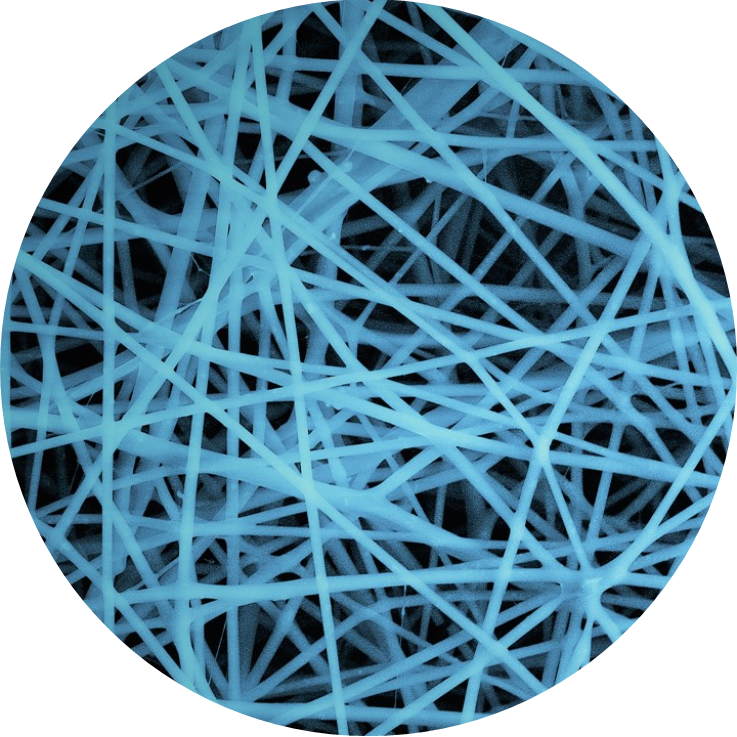
Application Note: Electrospinning of Collagen
Electrospinning is an extremely versatile technique for the production of micro and nanofibres, as a consequence electrospun fibres have been fabricated for a wide range of applications within regenerative medicine including tissue engineering scaffolds and wound dressings. All these applications are seeking to replace or repair tissue architecture and functionality. Thus, collagen which is a […]

ProColl Achieves ISO 13485:2016 Medical Devices Quality Management System Compliance with UKAS Accreditation
ProColl has received certification from URS for their ISO 13485:2016 quality management system, with accreditation from the United Kingdom Accreditation Service (UKAS) for the scope of manufacture of ProColl’s bovine and recombinant collagen products. ISO 13485:2016 is the medical industry’s primary medical device quality management system standard that ensures compliance with regulatory and customer needs. […]

Innovate UK Transformative Technologies Grant – Engineering Biology
ProColl have been awarded an Innovate UK Transformative Technologies grant in engineering biology, for its precision fermentation. ProColl’s type II and III recombinant human collagen will be available to innovative technology users by the turn of the year. Contact us today for more information or to get a quote for our innovative materials. Read the […]

In vitro Comparison of ProColl’s Recombinant Human Collagen with Bovine Collagen
Human tissues are composed of multiple proteins and molecules that interact with one another to optimise tissue function. Collagen is a major component and interacts with the tissue environment as more than just a structural protein. Medical, pharmaceutical and aesthetics industries use collagen, where its structure provides mechanical robustness, and its interactions provide beneficial effects. […]

ProColl makes using structured collagen affordable at last!
We are now able to offer Bovine Acid Soluble Collagen, the go-to product for structured & biocompatible volume use, at a significantly lower cost to you than ever seen before. Our single figure price per gram compares with the market’s typically more than £2,000 per gram for native collagen. ProColl’s scale manufacturing ensures consistent collagen […]
1
2
3

